Doping is a process of intentionally creating impurities into an semiconductor pure semiconductor for purpose of modulating its electrical properties . The impurities are dependent upon the type of semiconductor and the properties that it needs to have for its intended purpose. There are two types of semiconductor doping - p type and n-type . Let's have a look between the difference between the two .
| p Type
|
n Type
|
|
|
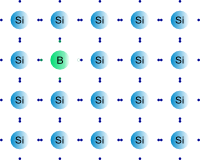
| In p type doping a donor with less valence electrons as needed in the semiconductor lattice is introduced . As a result they "accept" electrons from the semiconductor's valence band. This provides excess holes to the intrinsic semiconductor creating a p-type semiconductor .
|
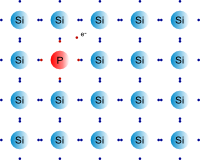
In n type doping a donor with excess valence electrons as needed in the semiconductor lattice is introduced as a result of which the free electrons from the semiconductor's valence band move freely in the semiconductor lattice thus creating a n type semiconductor .
|
| The p type semiconductor has greater hole concentration .
|
The n type semiconductor has greater electron concentration .
|
| The phrase 'p-type' comes from the overall positive charge of the semiconductor . In p-type semiconductors, holes are the majority carriers and electrons are the minority carriers.
|
The phrase 'n-type' comes from the negative charge of the electron. In n-type semiconductors, electrons are the majority carriers and holes are the minority carriers.
|
| For eg : Substituting a boron atom (with three valence electrons) for a silicon atom in a silicon crystal leaves a hole (a bond missing an electron) that is relatively free to move around the crystal
|
Substituting a phosphorus atom (with five valence electrons) for a silicon atom in a silicon crystal leaves an extra, unbonded electron that is relatively free to move around the crystal
|

